Latent Heat Is Best Described as
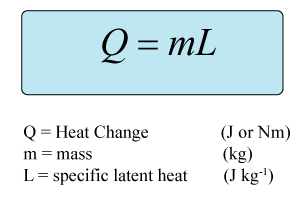
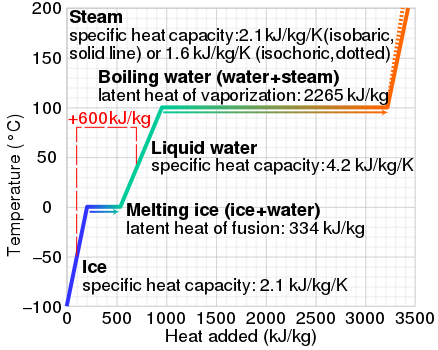
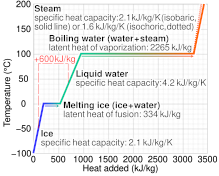
At 0C liquid water has 334 J g1 more energy than ice at. 334 kJ of energy.

Frost Protection Fundamentals Practice And Economics Volume 1
Asked May 27 2019 in Physics Space Science by tommys.
. Therefore the latent heat is more. 232 Latent Heat Storage. A phase-change material would normally be used to.
The best SHS liquid available is Water be-cause it is cheap and has a high specific heat. The following could be a source of latent heat. That is why they called the heat they transferred to the substance during phase changes latent heat ie.
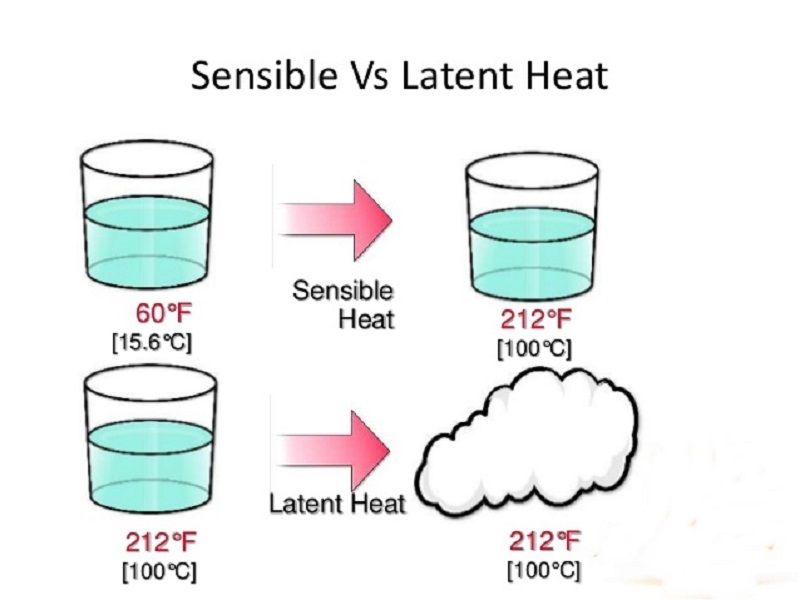
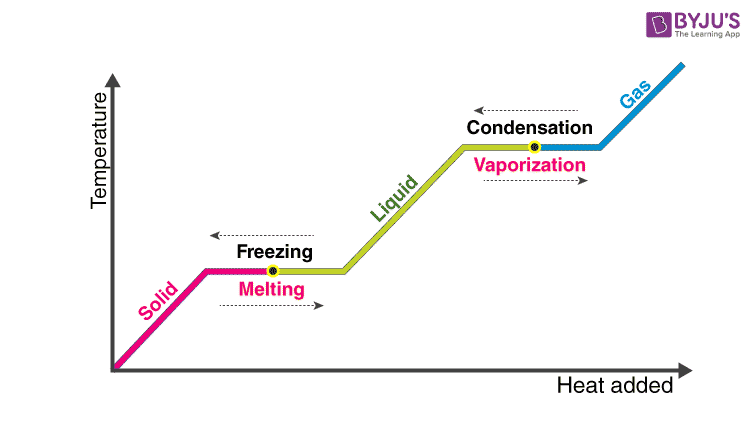
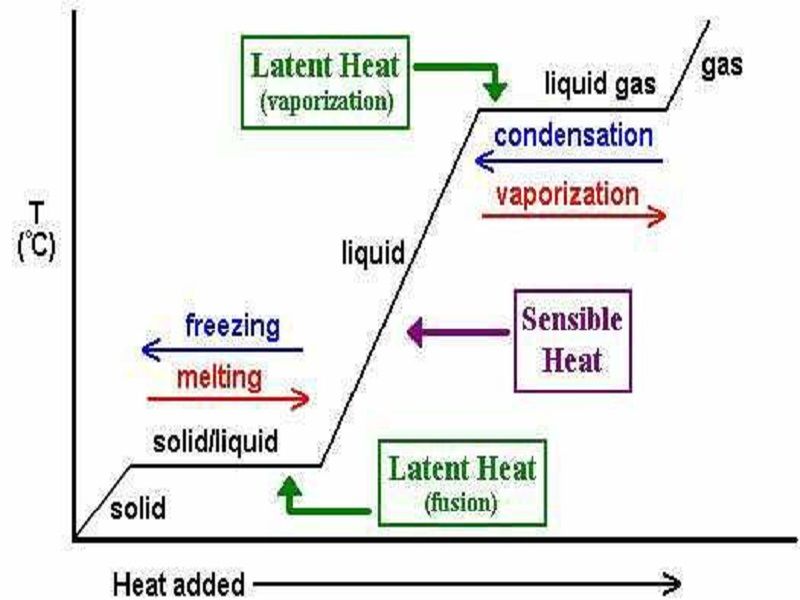
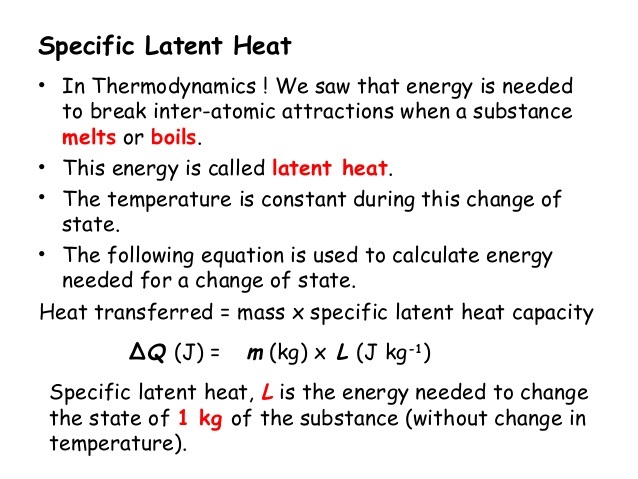
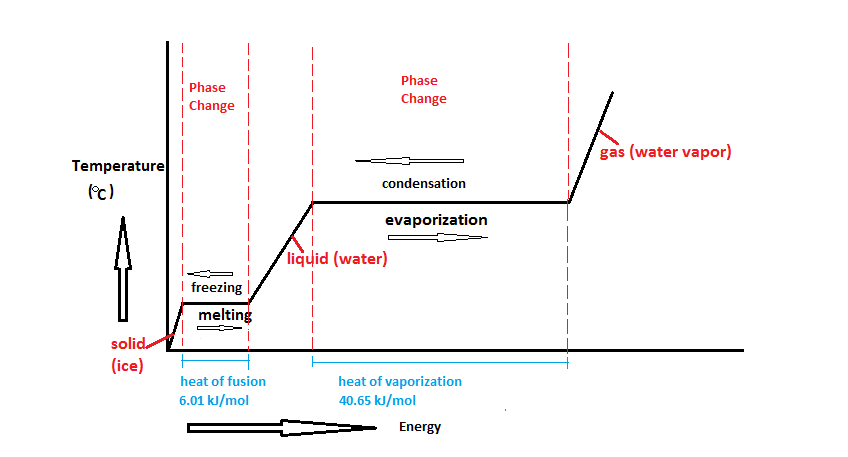
Latent heat of fusion also known as enthalpy of fusion is the amount of energy that must be supplied to a solid substance typically in the form of heat in order to trigger a change in its physical state and convert it into a liquid when the pressure of the environment is kept constant. Latent heat is the amount of energy that is either absorbed or released during a phase transition at a constant temperature. The quantity of heat absorbed or released by a substance undergoing a change of state such as ice changing to water or water to steam at constant temperature and pressure.
Also called heat of transformation. Several hours later a spike in temperature is recorded on the interior of the wall surface. The latent heat of vaporization of water means the amount of heat required to break the intermolecular forces and convert it into the gaseous phase.
As there is an increase in the pressure more heat is required to overcome the pressure force acting. The very real temperature increase described in 2 is a simple statistical anomaly. The temperature of the compressed gas leaving compressor can best be described as A.
Latent heat of sublimation. Asked May 27 2019 in Physics Space Science by tommys. Latent heat is the heat the results from an increase or decrease in the amount of moisture held by the air.
The heat absorbed or released per unit mass when water changes phase Explanation- During a phase shift a substances latent heat can be described as the heat or energy absorbed or released View the full answer. I suggest that your best bet would be to get someone with a process simulator who could generate a heating curve for your situation. I have never done a calculation like this and so cant be of very much assistance.
Latent heat energy absorbed or released by a substance during a change in its physical state phase that occurs without changing its temperature. A substance has a melting point of 20C and a heat of fusion of 36 104 Jkg. Some of the substances like naphthalene get directly converted.
Accepting the scenario as its been presented you would want to use the heat of solution rather than the latent heat. That associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of vaporization. The boiling point is 150C and the heat of vaporization is 72 104 Jkg at a pressure of one atmosphere.
A total of 334 J of energy are required to melt 1 g of ice at 0C which is called the latent heat of melting. Conductance is best described as. A Trombe wall is an example of which of the following passive solar heating system types.
The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion. Which change most likely caused the decrease in relative. A direct measure of the ability of any stated thickness of material to permit heat flow.
We now know that increasing temperature is linked to increasing kinetic energy of the molecules and that during an ideal phase change there is no increase in kinetic energy of the molecules. Humidity itself isnt latent heat but humidity contains. Latent heat is the heat released or absorbed by a body.
The amount of heat required by the unit mass of substance to vaporize from liquid to gaseous state or the amount of heat required to be removed from the unit mass of substance to condense from the gaseous to liquid phase is called as latent of vaporization denoted by lfu. Latent heat is a form of internal or potential energy stored by evaporated or melted water. Yet to the best of my admittedly limited knowledge no such release has ever been actually observed.
Changing this phase of matter is known as a phase transition. The reciprocating compressor frequently irn A- large industrial efrigeration systems 12. Phase of matter is the form of matter that has uniform chemical and physical properties all over the substance that is considered.
I suggest that the reason for this lack of observation is because no such energy as described in 3 exists. Sensible heat affects dry-bulb temperature whereas latent heat affects moisture content. However above 100 C oils molten salts and liquid metals etc.
Latent heat also known as latent energy or heat of transformation is energy released or absorbed by a body or a thermodynamic system during a constant-temperature process usually a first-order phase transition. Specifically its the amount of energy needed to cause a phase change for our purposes liquid-to-gas or gas-to-liquid without changing the actual temperature of a substance. Store heat in a a passive cooling or heating system.
Most of the heat absorbed by the suction gas is 10. Consider ice at -10 C. Latent heat is the stored heat energy in a substance used for changing the state of a substance.
The latent heat of condensation is the amount of heat removed so that water becomes ice. Base your answers to the following questions on the Earth Science Reference Tables the graph below and your knowledge of Earth science. The rock bed type storage materials are used for air heating appli-cations 2.
The boiling point is 150C and the heat of vaporization is 72 104 Jkg at a pressure of one atmosphere. A substance has a melting point of 20C and a heat of fusion of 36 104 Jkg. The sun strikes the outside of a 10 thick exterior stone wall.
The graph shows variations in air temperature and relative humidity for a spring day in Oswego New York.
What Is The Latent Heat Of Fusion Why Is It Called Latent Quora
Specific Latent Heat Physics Quiz Quizizz
What Is The Latent Heat Of Fusion Why Is It Called Latent Quora
9 Latent Heat Examples In Daily Life Studiousguy
Latent Heat Definition Types Formula Fusion And Vaporization
9 Latent Heat Examples In Daily Life Studiousguy

Latent Heat Formula Definition Concepts And Examples

Sensible Heat Vs Latent Heat Hvac Empire Engineering Facebook

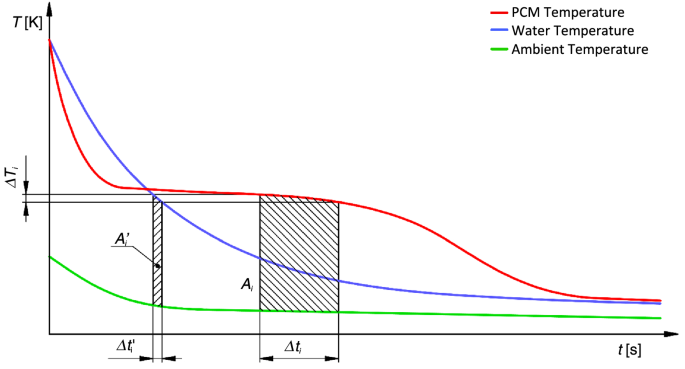
Experimental Investigation And Modelling Of A Laboratory Scale Latent Heat Storage With Cylindrical Pcm Capsules Scientific Reports

Melting Point Latent Heat Of Fusion Melting Point Of Metals
Icse Solutions For Class 10 Physics Specific Heat Capacity And Latent Heat A Plus Topper
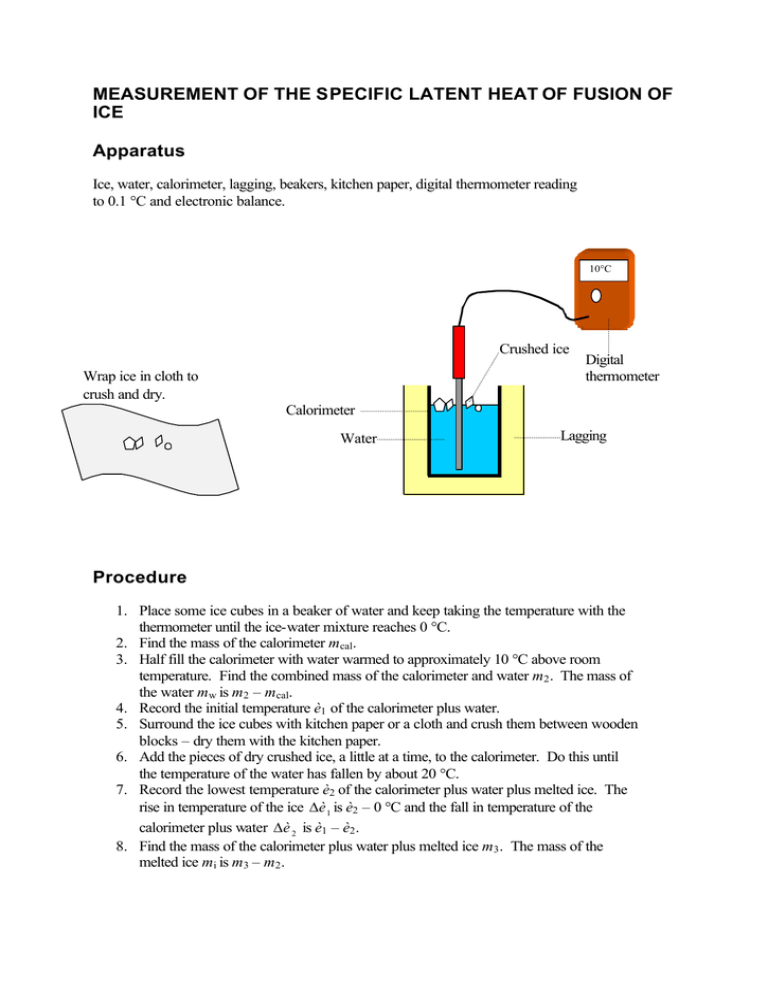
Measurement Of The Specific Latent Heat Of Fusion Of

Sensible Heat Vs Latent Heat And Temperature Control During The Phase Download Scientific Diagram



Comments
Post a Comment